Protein Slot Blot Protocol
- Protein Slot Blot Protocol Igg
- Cached
- Protein Slot Blot Protocol Assay
- Protocol: Dot Blot Checkerboard Titration Of Antibodies ...
- See Full List On Novusbio.com
- Protein Slot Blot Protocol Test
Dot Blot protocol A technique for detecting, analyzing, and identifying proteins, similar to the western blot technique but differing in that protein samples are not separated electrophoretically but are spotted through circular templates directly onto the membrane or paper substrate. Western blotting of proteins was introduced by Towbin et al. In 1979 and is now a routine and fundamental technique for protein analysis. Western blotting, also called protein blotting or immunoblotting, uses antibodies to identify specific protein targets bound to a membrane; the specificity of the antibody-antigen interaction enables a target protein to be identified in the midst of a.
Western blotting, also known as immunoblotting, is a well-established and widely used technique for the detection and analysis of proteins. The method is based on building an antibody:protein complex via specific binding of antibodies to proteins immobilized on a membrane and detecting the bound antibody with one of several detection methods.
Did you know: First described in 1979, the method of Western blotting has since become one of the most commonly used methods in life science research.
The Western Blotting Analysis Workflow:

Dot Blot The following protocol is a simplified alternative method, the Dot Blot, to traditional Western blotting for the detection of the presence or absence of a particular protein or bio-molecule in samples. Dot Blot differs from Westerns in that proteins in the samples are not resolved by size prior to blotting. Protein Blotting Workflow 6 7 Protein Blotting Guide Theory and Products Transfer The first phase of protein blotting is the transfer step, which involves moving the proteins from a solution or gel and immobilizing them on a synthetic membrane support (blot). Proteins can be transferred to membranes using a number of methods but the most.
Although the details of Western blotting protocols may vary from among applications, with adaptations to suit specific protein characteristics and the level of information required, they all follow some common basic steps. In this guide, we’ll explore these seven steps to Western blot.
Step 1. Sample Preparation:
The process begins with the sample of interest usually undergoing some degree of preliminary treatment before continuing to separation by electrophoresis. A sample can consist of a complex protein mixture, such as a cell or tissue extract, but it can also be a sample of purified proteins, such as a fraction from a purification procedure. The importance of good sample preparation cannot be stressed too highly. By understanding the nature of your starting sample and having a clear picture of the information you wish to derive from your Western blotting experiments, you increase your chances of a successful analysis. For more information around the ground rules of good practice in sample preparation, click here for Protein Sample Preparation from Cytiva.
Step 2. Gel Electrophoresis:
>
Electrophoresis is the second step, a commonly used method for separating proteins based on size, shape and/or charge. Electrophoresis is a separation technique based on the mobility of charged molecules in an electric field. It is used mainly for the analysis and purification of large molecules such as proteins or nucleic acids. This then aids the selection process when considering the conditions that will enable you to most effectively get the information you require from your specific analysis.
Although proteins may be separated and detected within gels by staining following electrophoresis, or may be subjected to the specialized process of 2-D gel electrophoresis for proteomics applications, we focus primarily on 1-D gel electrophoresis prior to transfer from gel to membrane for Western blotting in our handbook here. We have also considered some of the most important variables when planning electrophoretic separations, such as whether to use native or denaturing conditions, the choice of the most appropriate gel density (acrylamide percentage) as well as recommendations for the most appropriate buffer system.
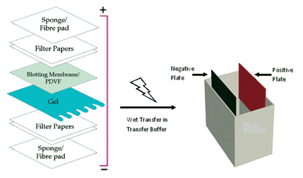
Step 3. Transfer:
>
On completion of protein separation by polyacrylamide gel electrophoresis (PAGE), the next step is to transfer the proteins from the gel to a solid support membrane, usually made of a chemically inert substance, such as nitrocellulose or PVDF. Blotting makes it possible to detect the proteins on the membrane using specific antibodies. The proteins transferred from the gels are immobilized at their respective relative migration positions at the time when the electric current of the gel run was stopped.
Related Techniques:
The term, 'Western blotting', was applied specifically to the transfer of proteins and their detection by antibodies and was presumably coined to indicate its relationship to a similar technique used for the detection of DNA; this method was called Southern blotting and was named after its inventor. This family of related techniques has continually expanded to include Northern blotting (RNA), Eastern blotting (post-translational modifications), Far Western blotting (protein:protein interactions), and Far Eastern blotting (lipid detection).
Click here for more information different approaches to Western blot transfer.
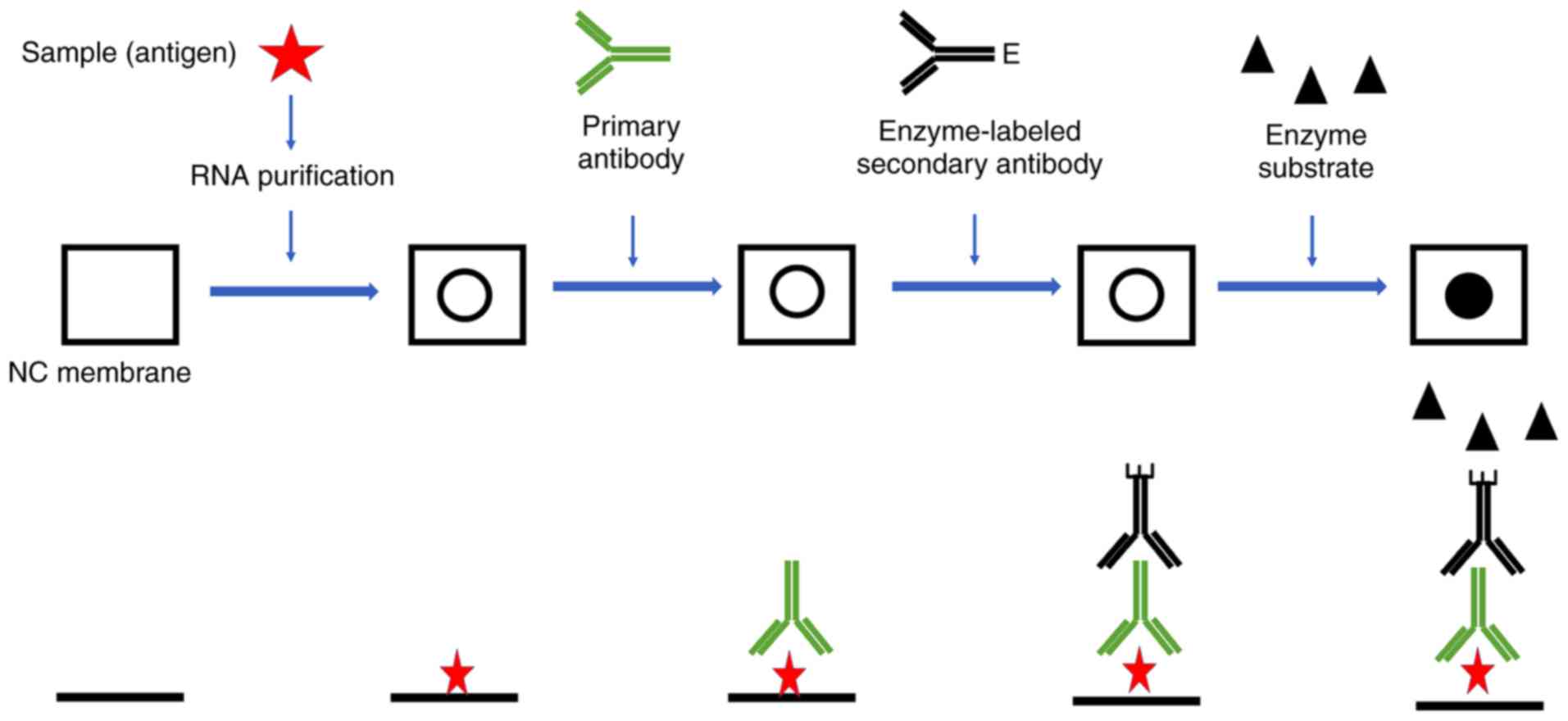
Step 4. Antibody Probing:
>
Protein Slot Blot Protocol Igg
Once your protein samples are separated and transferred onto a membrane, the protein of interest is detected and localized using a specific antibody. Usually, Western blotting protocols utilize an unlabeled primary antibody directed against the target protein and a species-specific, labeled secondary antibody directed against the constant region of the primary antibody. The secondary antibody serves not only as a carrier of the label, but is also a mechanism to amplify the emitted signals, as many secondary antibodies can theoretically bind simultaneously to the primary antibody. This is one of the most effective ways to maximize the potential sensitivity of the assay. For this reason, secondary antibodies are most often polyclonal and can target epitopes on the framework regions of the primary antibody; specificity is thus limited to species and immunoglobulin isotype. The signal emitted by the labeled secondary antibody is then measured and is proportional to the quantity of protein of interest present on the membrane.
With this highly specific immunodetection process, it is possible to reveal the presence of a very low quantity of a specific protein in a complex sample.

Cached
Step 5. Detection:
>
A variety of detection systems, based on chemiluminescence, chemifluorescence, fluorescence, chromogenic, or radioisotopic detection are available. Radioisotopic and chromogenic reagents have been widely used for many years, but have declined in popularity due to safety issues with handling radioactive isotopes and poor sensitivity with chromogenic reagents. As a result of these issues, enzyme-based chemiluminescent and chemifluorescent techniques, as well as direct fluorescence have been extensively developed and are now the preferred method for detection due to their improved sensitivity and wider dynamic range. Enzymatic detection methods, such as chemiluminescence and chemifluorescence require the addition of a reagent that emits light when it reacts with an enzyme conjugated to a secondary antibody. Fluorescence-based detection, on the other hand, requires no additional reagents after binding of the labeled secondary antibody. The most commonly used enzymatic detection system is chemiluminescence, based on antibodies conjugated to horseradish peroxidase (HRP) that catalyze the oxidation of luminol in presence of peroxide, resulting in light emission. HRP has several advantages over other enzymes such as alkaline phosphatase (AP). HRP can be easily conjugated to antibodies or streptavidin (which binds with extraordinarily high affinity to biotin, a commonly used tag, and can be used with different chemiluminescent reagents. Considerable efforts have been made to develop HRP-based detection reagents to obtain higher detection sensitivity, stronger light intensity and long-lasting signals. Fluorescence-based detection systems use a fluorescent entity, or fluorophore, directly conjugated to an antibody or streptavidin. The fluorophore can be excited using a light source of a specific wavelength causing light emission. Instead of adding a detection reagent, fluorescent signals can be directly detected with equipment, such as laser scanners, fitted with suitable light sources and emission filters.
Step 6. Imaging:
>
Protein Slot Blot Protocol Assay
The last step in the Western blotting workflow before data analysis is image capture. Enhanced chemiluminescence (ECL) is based on the reaction between an added luminol substrate and horseradish peroxidase (HRP)-labeled antibodies. In the presence of HRP, hydrogen peroxide catalyses the oxidation of luminol, a reaction that results in the emission of light. The light signal can then be detected on X-ray film or by digital imaging with a charge-coupled device (CCD) camera-based imager. When using fluorescence detection, a fluorophore is conjugated to the primary or secondary antibody. Light is emitted by the fluorophore after excitation via a specific wavelength of light. A photomultiplier tube (PMT) or a CCD can be used to collect and convert the emitted light to an electrical signal. The electrical signal is then digitized for image display and analysis.
Protocol: Dot Blot Checkerboard Titration Of Antibodies ...

See Full List On Novusbio.com
Step 7. Analysis:
>
Protein Slot Blot Protocol Test
Detection of signals, using either X-ray film, scanners, or a charge-coupled device (CCD) camera-based imager, results in one or more visible protein bands on the membrane image. The molecular weight of the protein can be estimated by comparison with marker proteins and the amount of protein can be determined as this is related to band intensity (within the limits of the detection system). In most applications, it is enough to confirm protein presence and roughly estimate the amount. However, other applications demand a quantitative analysis that defines protein levels in either relative or absolute terms. For a more thorough understanding of how to achieve precise quantitation in Western blotting experiments, click here.
Explore and learn more about our Western Blotting portfolio here.